Can 2 Different Ts Alleles of Sar1 Each Generate a Different Sec Phenotype?
- Research article
- Open up Access
- Published:
Isolation and characterization of new Saccharomyces cerevisiae mutants perturbed in nuclear pore complex assembly
BMC Genetics volume 3, Commodity number:17 (2002) Cite this article
Abstruse
Groundwork
Nuclear pore complexes (NPCs) are essential for facilitated, directional nuclear transport; however, the mechanism past which ~30 different nucleoporins (nups) are assembled into NPCs is unknown. We combined a genetic strategy in Saccharomyces cerevisiae with Green Fluorescence Protein (GFP) technology to identify mutants in NPC structure, assembly, and localization. To identify such mutants, a banking company of temperature sensitive strains was generated and examined by fluorescence microscopy for mislocalization of GFP-tagged nups at the not-permissive temperature.
Results
A total of 121 mutant strains were isolated, with near showing GFP-Nic96 and Nup170-GFP mislocalized to discrete, cytoplasmic foci. By electron microscopy, several mutants besides displayed an expansion of the endoplasmic reticulum (ER). Complementation analysis identified several mutant groups with defects in components required for ER/Golgi trafficking (sec13, sec23, sec27, and bet3). By directed testing, we institute that mutant alleles of all COPII components resulted in altered GFP-Nup localization. Finally, at least 9 unknown complementation groups were identified that lack secretion defects.
Conclusion
The isolation of sec mutants in the screen could reverberate a direct role for vesicle fusion or the COPII coat during NPC assembly; notwithstanding, only those sec mutants that altered ER structure affected Nup localization. This suggests that the GFP-Nup mislocalization phenotypes observed in these mutants were the indirect result of overproliferation of the ER and connected outer nuclear envelope. The identification of potentially novel mutants with no secretory defects suggests the distinct GFP-Nup localization defects in other mutants in the collection will provide insights into NPC structure and assembly.
Groundwork
Trafficking between the nuclear and cytoplasmic compartments is mediated by nuclear pore complexes (NPCs) [1, 2]. These big, proteinaceous structures are embedded in a nuclear envelope (NE) pore formed past fusion of the inner and outer nuclear membranes. The iii dimensional structure of NPCs from both Xenopus and budding yeast Saccharomyces cerevisiae is characterized by distinct substructures that are bundled with an 8-fold rotational symmetry [3]. 8 spoke-like structures form the central NPC core and are flanked on the cytoplasmic and nuclear sides by ring structures. Fibrils beetle from both the cytoplasmic and nuclear rings, with the nuclear fibrils gathered into a basket-similar structure past a last ring. The global architectures of the yeast and vertebrate NPCs are highly similar, although there may exist differences in the core ring structures [four]. Genetic and biochemical studies in yeast accept identified ~30 NPC proteins (termed nucleoporins, Nups) that incorporate the estimated ~44 MDa structure [5]. Recent proteomic assay has determined that mammalian NPCs are also comprised of ~thirty different proteins [half dozen]; notwithstanding, the overall size of the vertebrate NPC is predicted to be larger [three, vi, 7, viii]. Despite their size differences, yeast and vertebrate NPCs are responsible for identical transport reactions, and therefore, both structures are predicted to be functionally equivalent [9].
NPC assembly is likely to exist a highly regulated process that coordinates membrane fusion with the coincident associates of peripheral nucleoporins. Biogenesis of NPCs takes place throughout the prison cell cycle in all cell types. De novo associates into intact NEs occurs during prison cell and nuclear growth to maintain a constant NPC density [10]. In cells undergoing an open mitosis, NPCs are also re-assembled with the formation of the NE at the finish of mitosis [11]. The mechanisms controlling membrane fusion during pore germination and the steps for assembly of the distinct NPC substructures take non been fully elucidated. Agreement the molecular pathway of NPC biogenesis volition be a critical footstep in determining the mechanism of NPC role.
Current models for NPC assembly are based on a combination of in vivo and in vitro studies. Experiments using immuno-fluorescence in vertebrate cells are commencement to order the recruitment and timing of incorporation of individual nucleoporins during post-mitotic re-assembly [12–15]. Additionally, in vitro assembly studies with Xenopus egg or mitotic cell extracts take revealed several intermediates in NPC associates and demonstrated a requirement for an intact double nuclear membrane prior to post-mitotic NPC associates [16–19]. The modular nature of the NPC structure has also suggested that subcomplexes of detached Nups may initially form and provide edifice blocks for biogenesis. In fact, Hurt and coworkers have recently demonstrated the in vitro assembly of a Nup subcomplex from entirely recombinant proteins [20]. Given the structural and functional similarities between vertebrate and yeast NPCs and the insertion of both into a pre-existing NEs, it is likely that NPC biogenesis occurs via a similar mechanism in all organisms.
We and others take focused on genetic analysis in the budding yeast S. cerevisiae to understand the mechanism of NPC biogenesis. This approach has mostly involved reverse genetics wherein mutations in a gene encoding a known nucleoporin are evaluated for NPC structural and functional changes, and several mutants that induce massive morphological perturbations take been characterized [21–24]. However, the appearance of dark-green fluorescence protein (GFP)-based technology has recently allowed forward genetic strategies. Using GFP-tagged NPCs, we designed live jail cell assays to monitor NPC dynamics and assembly rates [25]. Moreover, to isolate factors required for proper NPC assembly and localization, nosotros developed a novel genetic screening strategy. The approach is based on the power to limited functional GFP-nucleoporin fusions, and the assumption that NPC assembly/mislocalization mutants expressing a GFP-nucleoporin volition take singled-out fluorescence properties every bit compared to wild blazon cells. Our previous studies used fluorescence-activated jail cell sorting to isolate mutants and identified a nup57 mutant with diminished incorporation of the GFP-Nup49p at the NPC [26]. As Nup57p and Nup49p direct interact in the NPC structure [27, 28], this provided a powerful strategy for pinpointing nearest neighbor interaction requirements. We have at present extended this analysis and employed classic temperature sensitive mutant collection with a strain expressing multiple GFP-tagged nucleoporins. This modified arroyo was designed to isolate mutants that bear on global NPC assembly. We written report here the complete mutant screen identifying 121 individual mutants that subdivide into at least 11 complementation groups. Further analysis of mutant subgroups has revealed potential mechanisms for both directly and indirect perturbation of NPC assembly and localization, and indicates that this process requires multiple factors
Results
Rationale for a genetic screen to place temperature sensitive mutants that adjy NPC assembly and localization
Work in our previous studies suggested that GFP-based screening for NPC assembly mutants (designated npa) was a powerful approach. This strategy was based on the ability to functionally limited GFP-nucleoporin fusions, and the assumption that NPC assembly/mislocalization mutants expressing a GFP-nucleoporin would have distinct fluorescence properties [26]. In wild type yeast cells, fluorescence microscopy localization of proteins at NPCs appears as punctate staining of virtually the unabridged nuclear rim circumference (see Effigy ii). Mutants perturbing GFP-Nup localization could include cells with 1) NPC clusters in NE subregions, 2) mislocalization of the GFP-Nup or aggregated NPC subcomplexes to the cytoplasm or nucleus (due to either a cake in new assembly or increased instability of existing NPCs), 3) mislocalization of the GFP-Nup due to indirect perturbation of the ER and NE membrane, or four) decreased GFP-Nup incorporation followed by turnover resulting in cells with macerated total fluorescence. Given the lack of cognition about mediators of NPC associates and localization, we aimed to bias the genetic strategy towards those with global assembly defects. We predicted that factors required for total NPC biogenesis would be essential, equally functional NPCs are absolutely required for nuclear transport. Thus, a primary modification of our original strategy was to generate a bank of temperature sensitive (ts) mutants from a parental strain expressing GFP-Nup(s). Examining the direct GFP-Nup fluorescence of each member of the banking concern would isolate potential npa strains. 2d, nosotros sought to preferentially isolate mutants that decrease overall NPC number versus inhibiting the incorporation of the single GFP-Nup. Nosotros predicted that co-expression of multiple GFP-Nups that localize to distinct NPC substructures would permit identification of mutants with global defects.
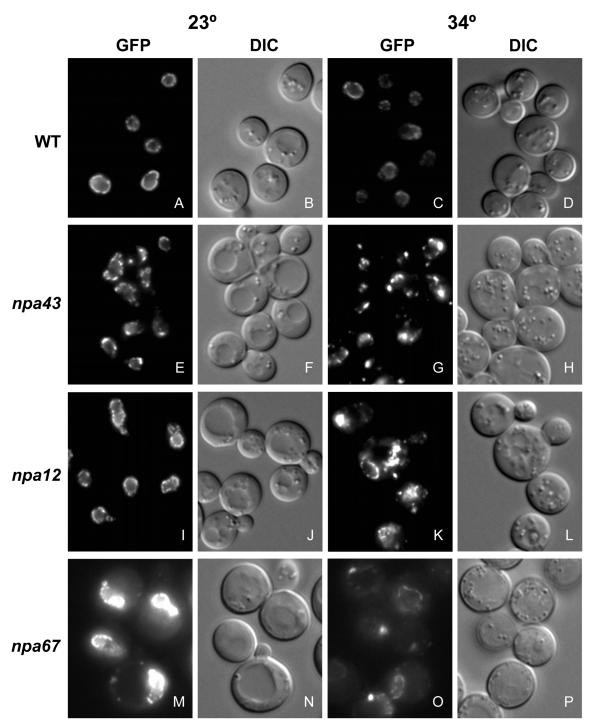
GFP-Nup localization in npa mutants. Isolated assembly mutant strains along with the parental strain were grown to early log phase at 23° then shifted to the not-permissive temperature of 34° for 4 hours. Localization of GFP-Nic96p and Nup170p-GFP was visualized by direct fluorescence microscopy, and all GFP images were collected for the same exposure time. WT cells (SWY2089) maintain a characteristic punctate nuclear rim staining, indicative of NPC localization, throughout the temperature shift. In contrast, the mutants display diverse localization phenotypes including NPC clusters (npa43, G), cytoplasmic and nuclear foci (npa12, K), and a diminished GFP-Nup signal (npa67, O). Note that while Nup-GFP localization is already affected at 23° in npa43 (E) and npa67 (One thousand), the phenotype is exacerbated at 34° (One thousand and O).
For such a screen, haploid strains expressing chromosomally integrated GFP fusions to both NIC96 and NUP170 were generated (see Materials and Methods). NIC96 is an essential nucleoporin and a member of multiple biochemically distinct NPC subcomplexes [29, 30]. NUP170, while not essential, is physically and genetically linked to a singled-out subset of nucleoporins [31–34]. The rationale for using NIC96 and NUP170 are several-fold. Nic96p and Nup170p are two of the most abundant nucleoporins [v, 31], the characterized nic96 and nup170 mutants do non result in gross morphological NPC perturbations (e.one thousand. NPC clusters, NE herniations, or membrane sealing of NPCs) [23, 31, 35], and in previous studies GFP-epitope tagging of Nic96p or Nup170p did not inhibit function [[26], our unpublished observations]. Finally, the encoded proteins are associated with distinct NPC subcomplexes [2], and we speculated that true mislocalization mutants would require disruption of both sets of subcomplexes and more probable reverberate defects in global NPC structure and assembly.
In the doubly tagged GFP-nic96 nup170-GFP strains, both GFP-Nic96p and Nup170p-GFP were targeted to the NE and showed punctate rim-staining feature of NPC localization (Effigy 2). Even so, the combination of these two protein fusions in a haploid strain resulted in a ts phenotype at 37°. The ts phenotype was not, however, accompanied by mislocalization of GFP-Nups after growth for vi hours at 37°. The strain was viable at 34° and doubling times were similar to wild type cells at 34°. We predicted this sensitized background might farther bias the screen toward mutations that perturb NPC construction.
Isolation of npa mutants
Wild type GFP-nic96 nup170-GFP haploid strains were mutagenized to generate a bank of ts mutant strains (Figure 1A; meet Materials and Methods). To identify mutants from this bank, individual ts strains were visually screened for GFP-Nup localization by fluorescence microscopy after shifting cultures to the not-permissive temperature (34°) for 4–vi hours. A total of 121 private npa strains were identified in which the GFP-Nup bespeak was altered. The mutant phenotypes were largely penetrant with all cells from a mutant strain responding identically. None of the cells in a mutant population had retained a completely normal appearance when assayed. Although most mutants had relatively normal growth rates at the permissive temperature, many showed signs of cell stress as indicated by large cell and vacuole size (Figure ii, DIC panels). While our previous screen identified mutants with either a diminished GFP signal (dim mutants) or NPC clusters [26], very few of the mutants isolated in this screen (v/121) showed a temperature dependent decrease in fluorescence intensity (Figure ii, compare panels A and C to M and O). Instead, most mutants lost the exclusive, punctate nuclear rim localization, the GFP-Nups were present in areas distinct from the NE and often in discrete foci (Figure ii, panels G and G). The majority showed wild type GFP localization at the permissive growth temperature (23°); yet some did show a weak mislocalization phenotype at 23° that was significantly enhanced when the cells were shifted to growth at 34° (Figure 2, panels E and G). Intriguingly, some of the mutants besides had an credible increase in GFP intensity (Figure ii, panel M). Nosotros speculate that this is due to changes in nearest neighbor interactions such equally those observed with GFP-Nup82p in a nup57 mutant [26].

Genetic strategy and isolation of npa mutants. A. Menstruum nautical chart of the genetic strategy used to isolate NPC assembly mutants (npa). B. Results of complementation testing between MAT a and MATαnpa mutants. ND, non determined.
To decide the number of independent mutant genes isolated, complementation analysis of the ts phenotype was performed. Based on pairwise crosses of mutants with reverse mating blazon, but one-third (37/121) of the mutants were initially catalogued into viii different complementation groups (Effigy 1B). The remaining two-thirds (84/121) of the mutants could non be assigned to a complementation group. Subsequent backcrossing and isolation of opposite mating type strains for the some of the mutants allowed definition of an additional complementation grouping. Still, the bulk of the mutants were apparently unique with only single mutant alleles isolated. This suggested that the screen was not saturating. Overall, the large number of independent npa mutants (potentially 87) was surprising and suggested that a number of singled-out proteins tin bear upon NPC assembly and localization.
Nup localization analysis in npa strains
To investigate the effects on NPCs more directly, we first targeted members of the largest independent complementation groups for analysis. The mutants displaying the strongest GFP-Nup mislocalization phenotypes were backcrossed. In each case, the GFP-Nup phenotype segregated 2:ii and was genetically linked to the ts phenotype indicating a single mutation was responsible for both. Interestingly, mutant alleles in the two largest complementation groups showed a like pattern of GFP-Nup mislocalization. To investigate NPC construction more closely in npa1-one and npa2-one mutants, logarithmically growing cultures were shifted to the non-permissive temperature and assayed for GFP-Nup localization by direct fluorescence microscopy at diverse times after the shift. The parental strain, SWY2089, maintained wild-type nuclear rim localization during growth at 34° (Effigy 2, panels A-D). In dissimilarity, both mutants showed a temperature dependent mislocalization of GFP-Nic96p and Nup170p-GFP over a 4 hour time form (Figure 3). Both npa1-one and npa2-1 cells ceased growing after iii–iv hours at the non-permissive temperature. During this time they completed 1 or 2 prison cell divisions. Previous studies of nic96 mutants accept shown that cells may undergo 2 to iii cell divisions with no apparent NPC assembly. Thus, the more rapid onset of growth arrest in npa1 and npa2 mutants suggested that the ts phenotype was not caused exclusively by a decrease in NPC number and nucleo-cytoplasmic send capacity [23, 35] and may be due to other cellular effects. Other members of the npa1 and npa2 complementation groups showed similar phenotypes (data not shown). In addition, Western assay showed no decrease in GFP-Nup protein levels in arrested npa1-1 cells indicating GFP protein stability was not affected (data non shown).
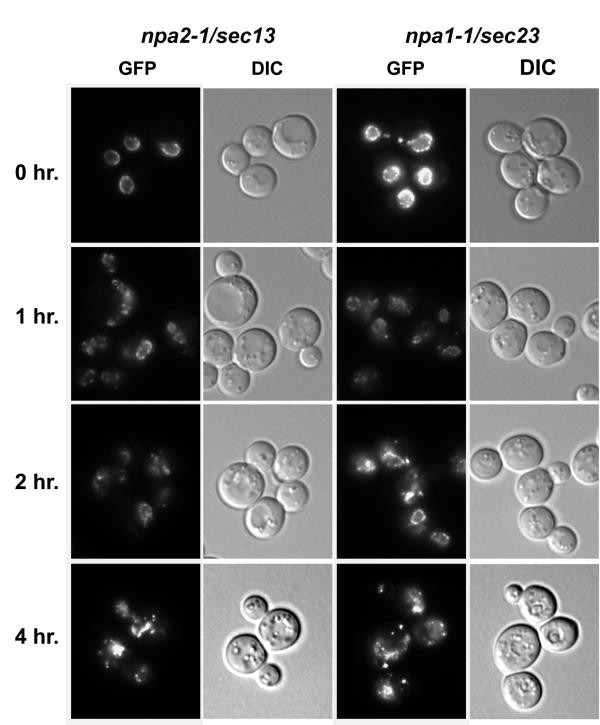
GFP-Nup localization in npa2/sec13 and npa1/sec23 mutants. Isolated mutant strains npa2-1/sec13-G176R and npa1-1/sec23-S383L were grown to early log phase at 23°, and then shifted to 34° for the indicated times. Localization of GFP-Nic96p and Nup170p-GFP was visualized by direct fluorescence microscopy. All GFP fluorescence images were taken for the same exposure time.
1 of our original predictions was that labeling multiple Nups with GFP would identify mutants with defects in the formation of the entire NPC structure. To determine if additional, not-GFP tagged Nups were mislocalized in these cells, nosotros used indirect immunofluorescence (IF) microscopy with antibodies directed against either Nup116p or Pom152p. Like GFP-Nic96p and Nup170p-GFP, the localization of Nup116p and Pom152p changed from a punctate, nuclear rim staining to cytoplasmic foci when shifted to the non-permissive temperature (information non shown).
Ultrastructural analysis of mutants by sparse department electron microscopy
Thin-section electron microscopy (TEM) was conducted to evaluate NPC, NE and cellular morphology. After staining with osmium tetroxide and uranyl acetate, NPCs in wild-type/parental cells were represented by electron dumbo structures spanning the pore (Effigy 4). In npa1-1 and npa2-1 cells, several distinct perturbations were noted. At the permissive temperature, npa1-ane cells showed nuclear invaginations. In Figure 4E, a single cell cantankerous-section showed multiple layers of NE containing NPCs. However, the GFP-Nup localization appeared normal in these cells at 23° (Figure 3). After shifting to the non-permissive temperature of 34° for 4 hours, in that location was a dramatic change in structural morphology in both npa1-i and npa2-1 mutants. Most hitting was the overproliferation of outer nuclear/endoplasmic reticulum (ER) membranes. This proliferation was oftentimes observed originating from the nucleus and resulted in a regular honeycomb-similar array of ~100 nm bore tubules (Effigy 4C and 4F). Although it was non e'er possible to definitively identify a nucleus in each department (Figure 4F, for case), NPCs were present in the envelope juxtaposed to the nucleus despite the expanded membranes (Figure 4C and 4D). Electron dense structures resembling NPCs were likewise embedded in membranes distinct from the nucleus/NE (Figure 4F). This most likely corresponded with the cytoplasmic foci observed by fluorescence. We did not find whatever evidence of cytoplasmic annulate lamellae formation, although nuclear invaginations sometimes resulted in juxtaposed double membranes containing NPCs (Effigy 4F). In that location were also no structures resembling the intra-nuclear annulate lamellae observed in some nup mutants [24, 36]. Samples analyzed by TEM over a fourth dimension course after shift to 34° showed that the membrane proliferation paralleled the GFP-Nup mislocalization seen by fluorescence microscopy (data not shown). This further suggested that the GFP-Nup mislocalization and TEM membrane proliferation were the consequence of the same process.
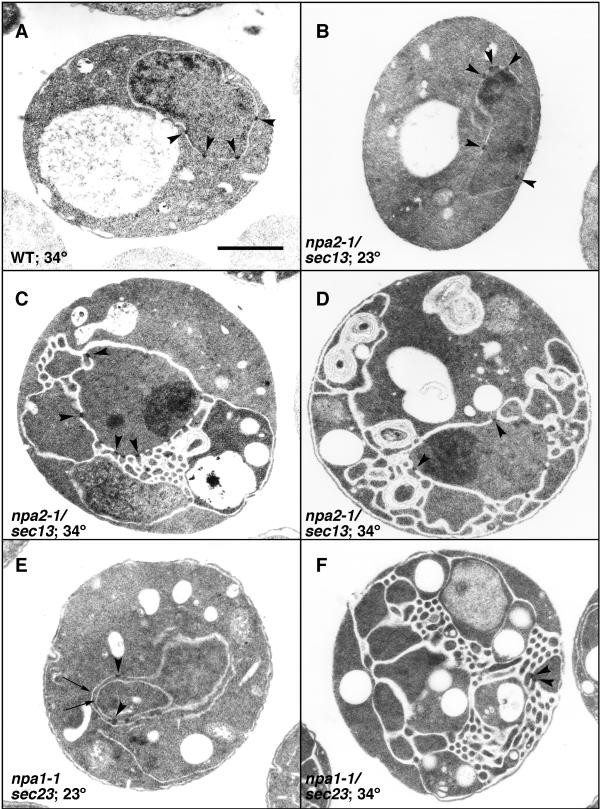
Sparse section electron micrographs of npa2/sec13 and npa1/sec23 mutants. Cells growing in early log phase were shifted to the permissive (B and E) or the non-permissive (A, C, D and F) temperature for iv hours before processing for TEM. Arrowheads denote NPC structures. The arrows in East evidence multiple layers of NE. Bar equals one μm.
Identification of mutants
To place the mutated genes, a yeast genomic library was screened for complementation of the recessive ts phenotypes of representative mutants. Multiple genomic inserts capable of rescuing the ts growth defect were isolated for each strain. Sequencing and comparison to the Saccharomyces Genome Database identified a minimal overlapping region on chromosome XII for the npa2-ane complementing plasmids, and on chromosome 16 in the npa1-1 complementing plasmids. Both regions independent multiple open reading frames; nonetheless, nosotros focused immediately on a single cistron for each mutant, SEC13 for npa2-1 and SEC23 for npa1-1. SEC13 was commencement identified in a screen for secretory mutants and encodes a protein of the COPII coat, which is required for vesicle budding from the ER [37–39]. More recently, it has been shown that a fraction of the full cellular Sec13p co-fractionates with NPCs in a distinct subcomplex with Nup84p, Nup85p, Nup120p, Nup145Cp and Seh1p [twoscore, 41]. SEC23 was also identified in a screen for secretory mutants, and it, as well, is a component of the COPII glaze [37–39]. Co-purification of Sec23p with intact NPCs has not been reported [5]; however, Sec23p was recently isolated in Nup42p and Nup116p affinity chromatography strategies [42]. Expression constructs containing just SEC13 or SEC23 were transformed into the mutant strains and assayed for their ability to rescue growth at the non-permissive temperature. Both minimal constructs just restored growth of their respective strain (information not shown). Importantly, the GFP-Nup localization phenotype was too complemented (Figure 5).
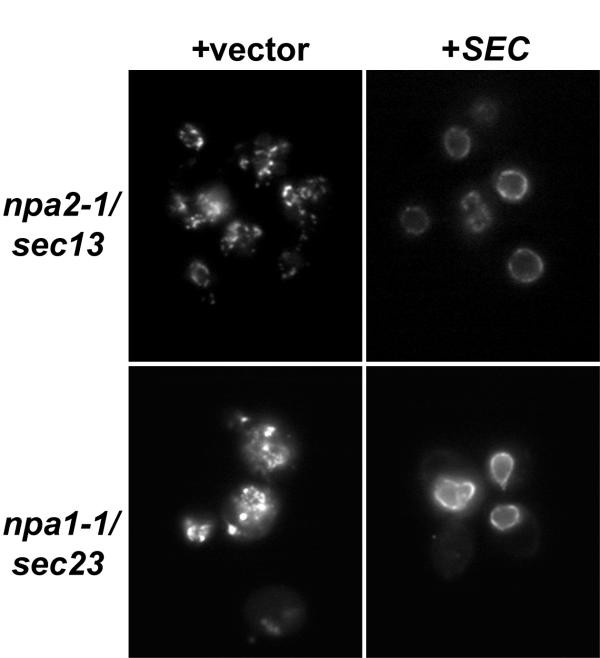
Rescue of GFP-Nup localization in mutants by transformation with SEC13 or SEC23 . Mutants npa2-1/sec13-G176R and npa1-one/sec23-S383L were transformed with an empty pRS315 vector (+vector), the vector containing SEC13 (npa2-1), or the vector containing SEC23 (npa1-1) (+SEC). Strains were grown for 4 hours at 34°C and GFP-Nup localization visualized as in Figures 2 and 3.
The power of SEC13 and SEC23 to complement the mutants strongly suggested that mutations in these genes were responsible for the observed phenotypes. However, it was still a formal possibility that the overexpression of these genes from a low re-create plasmid was sufficient for complementation. To eliminate this possibility, genomic Dna from each of the mutants was used as a template in PCR reactions to generate expression plasmids (meet Materials and Methods). Sequencing of the npa2-one/sec13 allele identified a single One thousand to A transition that changed a Gly to Arg at position 176 in the protein. To examination if this mutation was necessary and sufficient for the observed phenotype, the mutant plasmid was transformed into the original npa2-ane mutant and a sec13Δ covered past pSEC13 URA3. Expression of psec13-G176R complemented the otherwise lethal sec13Δ at 23°; still, its expression was not sufficient to restore growth at the non-permissive temperature to either the isolated npa2-1 mutant or sec13Δ (information non shown). DNA sequencing assay showed that the npa1-1/sec23 mutant was identical to the well-characterized sec23-1 (S383L) mutant allele [43].
The accumulation of not-secreted protein in mutants defective in ER to Golgi transport leads to proliferation of the ER [37, 44]. The membrane proliferation seen past TEM in npa1-one/sec23 and npa2-one/sec13 cells (Figure 4) and the coincident mislocalization of Nups (Figure 3 and information not shown) prompted us to test if Nups were accumulating at the ER. Logarithmically growing cultures of npa1-1/sec23 and npa2-1/sec13 were shifted to the non-permissive temperature as earlier and so fixed and candy for IF with polyclonal antibodies against Kar2p to visualize the ER [45]. The monoclonal antibody mAb414, which recognizes a subset of Nups distinct from Nic96p and Nup170p [46], was also used because the GFP fluorescence of the GFP-Nups was more sensitive to fixation when the GFP-Nups were mislocalized than when they remained at the nuclear rim (unpublished observation). In npa2-1/sec13 cells, the ER became punctate and formed discrete foci at afterward time points (Figure 6A). In contrast, npa1-1/sec23 cells rapidly accrue an interconnected ER network (Figure 6B). Expansion and changes in ER morphology were observed as early as one 60 minutes subsequently the temperature shift, and connected to increase in magnitude over the iv hour time course (Figure six). Although the localization patterns at belatedly fourth dimension points after temperature shifting were similar, the localizations of the ER protein Kar2p and the Nups were not overlapping.
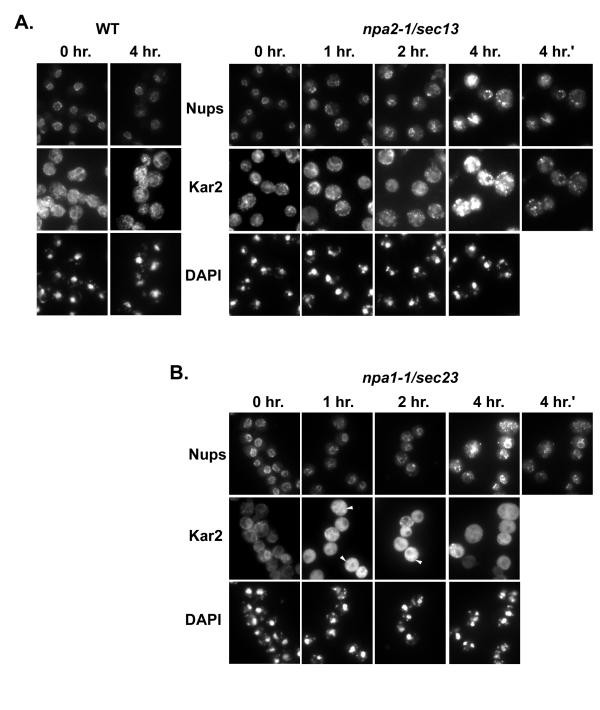
Comparison of nucleoporin localization with an ER lumenal poly peptide, Kar2, in npa2-1/sec13 and npa1-1/sec23 cells. Cells were shifed to 34° for the indicated times and processed for double-characterization IF with the monoclonal antibiotic mAb414 (Nups) and the rabbit polyclonal anti-Kar2 antibody. As the GFP-Nups were all the same present in these cells, the Nup signal resulted from a combination of GFP-Nic96, Nup170-GFP and the Nups recognized by mAb414. A. Wild-type (WT) and npa2-1/sec13 panels are shown where the 0, 1,2, and 4 hr. time points are exposed for the same fourth dimension. The 4 hr.' panels bear witness the aforementioned prison cell field as four 60 minutes. with decreased exposure times. B. Panels showing anti-Nup signal in npa1-1/sec23 cells were collected for the same exposure times as in A. Kar2 exposure times are 1 quarter of those in A, as the signal was brighter in these cells. Arrowheads denote cells in which the expanded ER network is virtually axiomatic.
Testing other COPII coat and secretory pathway components for npa groups
The screen for NPC assembly/mislocalization mutants identified 2 genes that are office of the COPII coat, SEC13 and SEC23. To test if other members of the COPII coat complex were isolated in the screen, conditional alleles in SEC16, SEC24, SEC31, SAR1, and SEC12 were kindly provided by other investigators, and tested straight for their effect on NPC localization. The first four genes encode proteins, which forth with Sec13p and Sec23p, grade the coat while Sec12p is required to recruit and activate Sar1p on the ER for glaze assembly [39]. Complementation analysis between these mutants and those isolated in the screen failed to identify any of these. Yet, when the genes encoding GFP-tagged Nups were crossed into these mutant backgrounds, all displayed GFP-Nup mislocalization under not-permissive atmospheric condition (Figure 7 and data non shown). The blueprint of mislocalization in each instance was similar to that observed in the isolated sec13 and sec23 mutants.
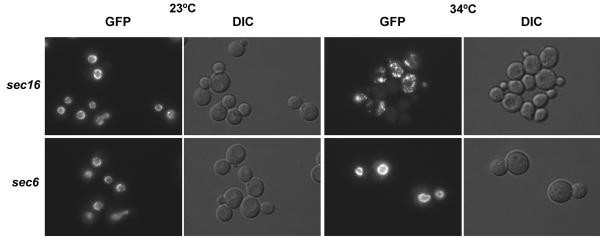
GFP-Nup localization in sec16 and sec6 mutants. Localization of GFP-Nic96p and Nup170-GFP in sec16-ii or sec6-4 mutant strains was analyzed afterward growth at 23° or afterward shifting to growth at 34° for four hours. Direct fluorescent signal was monitored.
Our continued efforts focused on cloning additional npa mutants by library complementation. This identified two more genes required for trafficking between the ER and Golgi. A genomic fragment containing BET3 complemented the ts phenotype of npa10, and npa3-1 was rescued by a library plasmid only when it contained full length SEC27 (run across Materials and Methods). Bet3p is function of a complex that binds to COPII derived vesicles and is required for vesicle targeting [47, 48]. Sec27p is a component of the COPI coat and required for Golgi to ER send [49, 50]. Similar SEC23 and SEC13 mutants, others take reported that mutants in BET3 and SEC27 upshot in an over-proliferation of ER membranes [49, 51].
To determine if the overlap between npa mutants and the secretory pathway was restricted to factor products affecting the ER, the sec6-4 mutant perturbing Golgi to plasma membrane transport [52] was crossed into the GFP-nic96 nup170-GFP strain. After 4 hours at 34°, the same conditions that crusade GFP-Nup mislocalization in the other assayed sec mutants, the GFP-Nups in sec6-4 remained localized to the NPC in the characteristic, punctate NE rim design (Figure vii).
Novel npa groups lack secretion defects
The initial isolation of multiple sec mutants from our screen that was designed to isolate mutants in NPC assembly and localization raised the question of whether whatever of the mutants were specific for defects in NPC assembly/structure. To pinpoint mutants that lacked secretion defects, we assayed each mutant from the collection for the ability to secrete the enzyme invertase in response to low glucose. Logarithmically growing cells were transferred to depression glucose media and incubated at the non-permissive temperature of 34° for 2.5 hours. When invertase activity from individual mutants was normalized to the activity of the parental strains, a range of relative activities were observed (Figure 8). With the exception of npa1-three and npa1-7, the sec23 (npa1), sec13 (npa2), and bet3 (npa10) mutants had less than l% of wild-type invertase activity (Figure 8A). The npa3/sec27 mutants displayed near wild-type invertase activity indicating that forward trafficking through the ER was not significantly disrupted. This is consistent with secretion results reported for other sec27 alleles [49, 53]. It is important to note that despite the lack of secretion defect, the npa1-three/sec23, npa1-7/sec23, and npa3-one/sec27 mutants each had perturbations in ER structure similar to those of npa1-1 and npa2-1 (data not shown). The npa1-iii/sec23 and npa1-7/sec23 mutants may be unique alleles that preferentially touch NPC assembly versus secretion. For the unidentified, potentially novel, mutants, approximately 60% (56/96) had less than one-half of wild-type invertase activity. This suggests secretion may exist impaired in these mutants. Alternatively, these mutants may have defects along the pathway leading to transcriptional up-regulation of invertase (SUC2) in response to low glucose [54]. Most importantly, 25% (25/96) of the mutants had at least 75% or greater of wild-type activity (Figure 8B). The lack of secretion defects in some of the mutants indicates that non-nucleoporins are being trafficked properly when Nups are mislocalized.
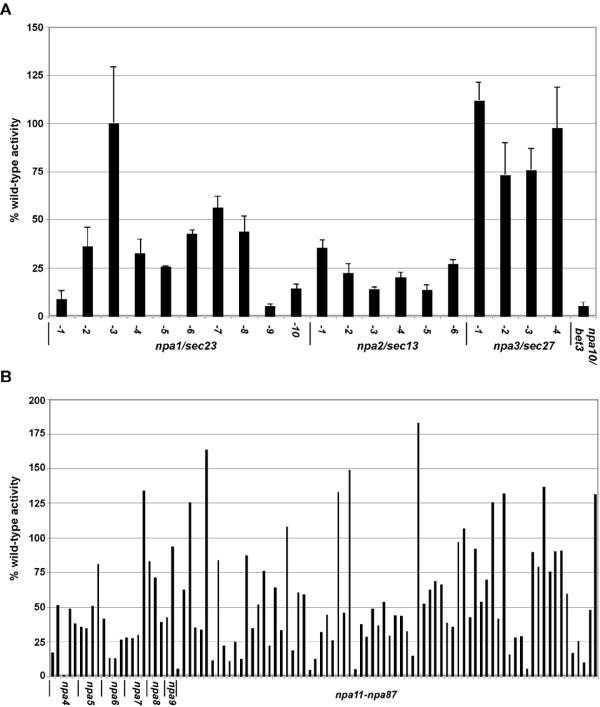
Secreted invertase activity of npa mutants. The ability of mutants to secrete the enzyme invertase at the not-permissive temperature (34°) in response to low glucose was determined. Absolute values were normalized to the wild-type control assayed at the same time and plotted as % wild-type activity. A. The average % activity and standard departure of npa mutants in the secretory pathway. B. Invertase action from 96 of the remaining 100 uncharacterized npa mutants was assayed. In strains assayed multiple times (15/96), the boilerplate activity from independent assays is presented.
Give-and-take
Nosotros take used a genetic strategy to identify factors required for proper NPC biogenesis. By creating a bank of ts mutants in a doubly tagged GFP-nic96 nup170-GFP S. cerevisiae strain and individually screening the mutants at the non-permissive temperature, we identified 121 ts mutant strains which fail to correctly localize the GFP-Nups at NPCs and/or the NE rim. In add-on to the mislocalization of GFP-Nic96p and Nup170p-GFP, Nup116p, Pom152p and Nups recognized by mAb414 were too perturbed in the mutants characterized to date. These Nups are localized throughout the NPC structure [1] and indicate a global defect in NPC assembly/construction. Complementation analysis indicated the mutants were all recessive and brutal into at least xi groups. In comparison to our previous genetic screen with a strain expressing only GFP-Nup49p [26], these results propose that increasing the number of GFP-Nups every bit reporters for NPC integrity effectively increases the likelihood of obtaining a panel of mutants defective in global NPC associates, stability, and localization.
Based on the GFP-Nup localization and genetic analysis, a range of mutant phenotypes and complementation groups were catalogued. Several of the complementation groups independent multiple independent alleles (npa1-9), and within a unmarried complementation group the strengths of the defects were varied. However, there was only one allele for the bulk of the complementation groups, representing potentially npa10-87. This suggests that the mutagenesis was non saturating, and it is probable that mutations in additional genes that function in NPC biogenesis would exist isolated past repeated application of this screening strategy. In add-on, the large number of potential total groups may reflect roles for many factors in proper NE/nuclear morphology and NPC biogenesis. We have identified most 30% of the groups past complementation-based cloning. The results thus far indicate the screen is specifically enriched for NE/NPC specific furnishings versus nonspecific mutants that may affect GFP-Nup protein stability or levels (polymerases, transcription/translation factors, chaperones) (this study and unpublished results). The ts defects in individual npa mutants may represent failures in assembly, changes in NPC localization, or perturbations in the stability of pre-existing NPCs.
Here we focused on characterizing the largest complementation groups. The two largest were allelic with SEC23 and SEC13, genes encoding known mediators of vesicular trafficking between the ER and Golgi [39]. The GFP-Nup mislocalization defects in the sec23 (npa1) and sec13 (npa2) mutants correlated with distinct ER membrane perturbations. We also identified npa3 equally sec27 and npa10 every bit bet3. Taken together, the recovery of a big number of npa mutants that encode components of the ER-Golgi secretory pathway indicate that this is a "hot spot" for mutations that cause perturbations in GFP-Nup localization.
Extensive and elegant studies have documented distinct changes in membrane morphology in mutants with arrest points in the secretory pathway. Three morphological classes include sec mutants with extended networks of ER membrane, those with accumulated Golgi, and a third with accumulated 80–100 nm secretory vesicles [37]. The morphological perturbations direct correlate with singled-out secretory pathway blocks: ER-Golgi, intra-Golgi, and Golgi-plasma membrane [44]. A recent written report in Due south. pombe has also shown that sec mutants can impact NE and prison cell bicycle progression [55]. In addition to sec13, our screen also identified mutant alleles of SEC23, SEC27 and BET3. The mislocalization of GFP-Nups in sec13 and sec23 mutants was coincident with the germination of extensive honeycomb networks of ER. Interestingly, nosotros constitute by direct testing that mutant alleles corresponding to the genes for all known COPII coat components resulted in contradistinct GFP-Nup localization. Based on reports from others, all of these mutants have an over-proliferation of ER membranes [37, 44, 49, 51, 56, 57, 58, 59]. However, over the same time course, our direct testing of one sec mutant with a defect in a after secretion footstep did not testify perturbations (e.g. the sec6-4 mutant that accumulates secretory vesicles [37] did non result in GFP-Nup mislocalization). Additionally, sec mutants with no apparent defect in invertase secretion (npa1-3/sec23, npa1-seven/sec23, and npa3-ane/sec27) had both perturbations in ER structure and Nup localization. This suggests that mutants which disrupt the ER will affect Nup localization and likely NPC assembly/structure.
Connections between Sec13p and NPC function have been previously reported. A fraction of the total cellular Sec13p is isolated in a NPC subcomplex containing Nup84p, Nup85p, Nup120p, Nup145p-C, and Seh1p (a Sec13-related poly peptide) [40, 41]. Hurt and coworkers have also reported that ts sec13 mutants evidence enhanced growth defects when combined with a nup85Δ mutant, and event in the mislocalization of GFP-Nup49p to NE clusters and cytoplasmic foci [41]. This correlates with our observations of GFP-Nic96p and Nup170p-GFP in our sec13/npa2 mutant. Withal, we did not discover clusters of herniated NPCs in sec13-G176R/npa2-ane cells suggesting the sec13-14 and sec13-34 alleles in the Hurt study are singled-out [41]. Precisely how Sec13p influences NPC structure/function remains to be elucidated.
The phenotypes observed in sec13 and sec23 mutants might indicate that COPII vesicles and/or a vesicular biogenesis step is required for NPC formation; however, there are at least two indirect mechanisms past which NPC structure may be changed in these mutants. Blocking the ER/Golgi pathway could titrate away the pool of Sec13p that is found at the NPC. Alternatively, the extensive ER proliferations that outcome are continued to the outer NE and could foreclose proper NPC localization. Given that the formation of the all-encompassing membrane networks in the sec13 and sec23 mutants was coincident with the GFP-Nup mislocalization, we favor i of the ii indirect models. We experience this conclusion is also supported by our ascertainment that the distribution of GFP-Nups was altered in mutants for all COPII coat components. However, non all of the COPII components co-fractionate with NPCs [5, half-dozen, 42]. Thus, there are COPII components that adjy GFP-Nup localization that practise non co-fractionate with NPCs. We conclude at that place is no absolute correlation between localization of a COPII component at the NPC and its ability to disrupt NPC structure/function. Instead, it is the sec mutants that alter ER structure that result Nup localization.
The defects that we have observed in GFP-Nup localization in various sec mutants are singled-out from recent reports characterizing a novel abort of secretion response in S. cerevisiae cells. Tartakoff and coworkers have proposed that mutants with a secretory block show altered nuclear transport capacities, changes in the nuclear localization of particular proteins, and potential perturbations of NPC composition [threescore, 61]. In our sec13 and sec23 mutants, nosotros do not believe NPC composition has inverse only rather that NPC localization has been altered past formation of extended ER networks.
Conclusions
Taken together, the work to date supports a framework wherein a network of in vivo interactions mediate NPC biogenesis, structural integrity, and proper localization in the NE. A complete understanding of the function of the NPC in nuclear send will require the identification of all the genes that regulate its assembly, stability, and localization. Although the npa/sec mutants characterized here are due to ER distortions, we predict that further assay of the other npa mutants and GFP-based genetic strategies will significantly contribute to the effort of identifying factors required for NPC biogenesis.
Methods
Yeast Strains
All yeast strains used in this study are listed in Table ane. Full general yeast manipulations were conducted by standard methods [62] with transformations by the lithium acetate method [63]. All strains were grown at 23° in YPD (yeast excerpt, peptone, ii% glucose) unless otherwise noted. The sequence encoding GFP was fused in frame to the 3' finish of the NUP170 open reading frame using the method of Baudin et al. [64]. To create a template for the polymerase chain reaction (PCR), the BamHI fragment of GFP S65T F64L [65] was first cloned into pRS316 [66]. The Deoxyribonucleic acid fragment for integration was generated by PCR using the following oligonucleotides: Nup170G (5'-GATCCAATTGAAAAGTACGTTAAGAACAGCGGCAATAATTTGGGGATTTGTTTCTACAAAGAAGCTGCGATGAGTAAAGGAGAAGAACTT-3') and Nup170V (5'-TTACTTACAGATTACGATCTGTTCAATGCATCAGAAATAGGAAGAAATTCAAAGCGGCATCAGAGCAGATTGT-3'). The resulting fragment was gel purified and transformed into SWY519. Correct chromosomal integration was confirmed by fluorescence microscopy and immunoblotting with anti-GFP antibodies (rabbit analogousness purified IgG; kindly provided by M. Linder, Washington Academy School of Medicine, St. Louis, MO).
Crossing a nup170-GFP:URA3 haploid isolate to SWY1695 generated doubly tagged GFP-nic96 nup170-GFP parental strains. The lys2 marker was introduced by crossing to YCH128. GFP-tagged strains of other known mutants were made by crossing to either parental strain SWY2089 or SWY2090.
Null (Δ) deletion strains of SEC13 and SEC23 were purchased equally heterozygous diploids from Research Genetics (Huntsville, AL). Each strain was transformed with a plasmid harboring the respective wild blazon gene in pRS316 (pSW1268 or pSW1269; see below) [66] and sporulated to isolate haploid secΔ deletion strains with the pSEC/URA3/CEN plasmid. To test the function of the isolated mutant alleles, the resulting haploid secΔ pSEC/URA3/CEN strains were transformed with the mutant sec alleles in a LEU2/CEN plasmid (pSW1264 or pSW1266; run into below). Strains lacking the wild type SEC/URA3/CEN plasmids were isolated by growth on media containing 5-fluoroorotic acid (5-FOA).
Plasmids
Plasmids with wild type SEC13 were generated with the following oligonucleotides (5' oligo sec13N5 with 5'-CCCGGGCTAGCACTTCAATGTT-iii'; 3' oligo sec13-3 with 5'-ACCATTGAGCTCTTCACTGATGAACTTCACC-three') using PCR and an isolated library plasmid as template. The PCR product was digested with SacI and XbaI and cloned into the SacI/XbaI sites of both pRS315 (pSW1264) and pRS316 (pSW1268) [66]. Plasmids with wild type SEC23 were constructed in the aforementioned fashion using specific oligonucleotides (5' oligo sec23F5 with five'-ATGAATATCTAGACCAGGGTGCC-3'; 3' oligo sec23-3 with 5'CCTTGGATCCGTAGTAAAGGCCACGCAG-three') and digestion of the PCR product with XbaI and BamHI to yield pSEC23/LEU2 (pSW1266) and pSEC23/URA3 (pSW1269). Sequence for the mutant sec13 and sec23 alleles were cloned using the same oligonucleotide pairs with genomic DNA from SWY2324 or SWY2325, serving every bit template. 2 independent PCR trials were completed for each mutant allele and cloned into the pRS315 vector [66]. Clones from each were obtained and both strands sequenced.
Mutagenesis and screening for mutants
Mutagenesis of SWY2089 and SWY2090 with ethylmethane sulfonate (EMS; Sigma, St. Louis, MO) was performed every bit described with the following modifications [67]. Showtime, cells were mutagenized for threescore, 75, and ninety minutes, followed by plating a fraction of the culture to determine prison cell viability at 23°. The remaining cells were stored in water at 4° for three days until the viability could be analyzed. The remaining mutagenized cell cultures were and then plated to obtain ~100,000 colonies from each strain. To achieve this number, we assumed that roughly one-half of the cells died during the 4° storage. Temperature sensitive mutants were isolated by replica plating the main to both 23° and 34°, and screening for those that failed to abound at 34°. To screen for GFP-Nup localization, temperature sensitive strains were inoculated individually in 2 ml liquid YPD cultures and grown at 23° for 12–16 hours before shifting to 34° for iv–6 hours. The majority of the strains were in logarithmic growth phase at the time of the temperature shift. GFP localization was determined by direct fluorescence microscopy of unfixed cells. Fluorescence screening was repeated 2 additional times on those with an altered GFP pattern to confirm the phenotype.
Mutant complementation assay
Complementation assay of the temperature sensitive phenotypes was performed by crossing all the MAT a mutant strains to all MATα mutant strains and selecting for diploids on media lacking lysine and tryptophan. Diploids were then replica plated back to YPD and assayed for growth at 34°. For direct testing, temperature sensitive mutants in genes involved in vesicular budding from the endoplasmic reticulum were obtained from others and crossed to all mutants isolated in the screen. Diploids were selected on appropriate minimal media, and and so assayed for growth at the non-permissive temperature of 37°. (The GFP-Nups did not cause a temperature sensitive phenotype in heterozygous diploids). Additionally, the sar1Δ crosses were grown on glucose to repress the plasmid borne copy of GAL-SAR1.
Microscopy
Live prison cell fluorescence and DIC microscopy was performed on an Olympus BX50 microscope using an UPlan 100X/ane.3 objective. Cells for photography were grown in media lacking histidine, as the HIS3 linked GFP-tag of GFP-nic96 showed some instability in the nup170-GFP background. Images were captured using a Dage-MTI CCD-300-RC camera with NIH Image 1.61 or a Photometrics CoolSnap HQ photographic camera with MetaVue software. Indirect immuno-fluorescence was performed as previously described [46]. Rabbit anti-Kar2 polyclonal antibodies [45] were used at 1:5000 and detected with Texas Red Caprine animal-anti-Rabbit secondary antibiotic. The monoclonal mAb414 [68] and anti-Pom152 monoclonal mAb118C3 [69] were detected with FITC Goat-anti-mouse secondary. Sparse section electron microscopy was conducted according to [36].
Molecular cloning
Selected mutants were backcrossed until the temperature sensitive phenotype segregated 2:2 and was shown to be linked to the GFP mislocalization phenotype. Identification of the mutant genes was accomplished by complementation of the temperature sensitive phenotype. The SWY2324, SWY2325, SWY2332, and SWY2333 strains were transformed with a LEU2/CEN yeast genomic library (ATCC, Manassas, VA). Transformants were grown for 48 hours at 23° and and then shifted at 34°. Multiple overlapping inserts capable of rescuing the temperature sensitive growth defect were isolated for each strain. Sequencing of the insert ends and comparison to the Southward. cerevisiae Genome Database identified a region on chromosome 16 for npa1-1 and npa1-2 and chromosome XII for npa2-1. Plasmids expressing only SEC23 or SEC13 were used to exam if these genes were necessary and sufficient for rescuing the phenotypes. Library inserts rescuing npa3-1 contained a region of chromosome Vii encompassing SEC27. A partial deletion of SEC27 from one of the inserts (pSW1386) was used to confirm that SEC27 was responsible for rescue of the growth defect. The temperature sensitive growth defect of npa10 was rescued past a region on chromosome Xi from YKR064W to the 5' end of MET1. The only essential factor in this region is BET3.
Invertase Assays
Mutants were grown at 23° overnight in 5 ml YPD to logarithmic phase. The equivalent of 1 ml of a 0.5 OD600 cell civilisation was washed into YP media containing 0.05% glucose and incubated at 34° for 2.5 hours. The incubation was concluded by add-on of an equal volume of 20 mM sodium azide. Cell pellets were washed once with 10 mM sodium azide then resuspended in 800 μl of 10 mM sodium azide. Assays of invertase activity were performed with 25 μl of each sample co-ordinate to [seventy, 71]. The action of each mutant was normalized to the activity of the parental strain assayed at the same time.
References
-
Rout MP, Aitchison JD: The nuclear pore complex as a send machine. J Biol Chem 2001, 276:16593–16596.
-
Ryan KJ, Wente SR: The nuclear pore complex: a protein machine bridging the nucleus and cytoplasm. Curr Opin Prison cell Biol 2000, 12:361–371.
-
Stoffler D, Fahrenkrog B, Aebi U: The nuclear pore circuitous: from molecular architecture to functional dynamics. Curr Opin Cell Biol 1999, eleven:391–401.
-
Yang Q, Rout MP, Akey CW: 3–dimensional architecture of the isolated yeast nuclear pore complex – functional and evolutionary implications. Mol Prison cell 1998, 1:223–234.
-
Rout MP, Aitchison JD, Suprapto A, Hjertaas One thousand, Zhao Y, Chait BT: The yeast nuclear pore complex: limerick, architecture, and send mechanism. J Cell Biol 2000, 148:635–651.
-
Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ: Proteomic assay of the mammalian nuclear pore circuitous. J Cell Biol 2002., 158:
-
Fontoura BMA, Blobel G, Matunis MJ: A conserved biogenesis pathway for nucleoporins: proteolytic processing of a 186–kilodalton precursor generates Nup98 and the novel nucleoporin, Nup96. J Cell Biol 1999, 144:1097–1112.
-
Miller BR, Forbes DJ: Purification of the vertebrate nuclear pore complex past biochemical criteria. Traffic 2000, 1:941–951.
-
Wente SR: Gatekeepers of the nucleus. Science 2000, 288:1374–1377.
-
Winey Thou, Yarar D, Giddings Thursday Jr, Mastonarde DN: Nuclear pore complex number and distribution throughout the Saccharomyces cerevisiae prison cell cycle past three–dimensional reconstruction from electron micrographs of nuclear envelopes. Mol Biol Cell 1997, 8:2119–2132.
-
Gant TM, Wilson KL: Nuclear Assembly. Annu Rev Prison cell Dev Biol 1997, xiii:669–695.
-
Bodoor K, Shaikh South, Salina D, Raharjo WJ, Bastos R, Lohka Grand, Shush B: Sequential recruitment of NPC proteins to the nuclear periphery at the end of mitosis. J Jail cell Sci 1999, 112:2253–2264.
-
Haraguchi T, Koujin T, Hayakawa T, Kaneda T, Tsutsumi C, Imammoto N, Akazawa C, Sukegawa J, Yoneda Y, Hiraoka Y: Live fluorescence imaging reveals early recruitment of emerin, LBR, RanBP2, and Nup153 to reforming functional nuclear envelopes. J Cell Sci 2000, 113:779–794.
-
Daigle N, Beaudouin J, Hartnell L, Imreh G, Hallberg E, Lippincott–Schwartz J, Ellenberg J: Nuclear pore complexes course immobile networks and have very low turnover in live mammalian cells. J Jail cell Biol 2001, 154:71–84.
-
Belgareh North, Rabut Thousand, Bai SW, van Overbeek Yard, Beaudouin J, Daigle Due north, Zatsepina OV, Pasteau F, Labas V, M Fromont–Racine, et al.: An evolutionarily conserved NPC subcomplex, which redistributes in part to kinetochores in mammalian cells. J Cell Biol 2001, 154:1147–1160.
-
Macaulay C, Forbes DJ: Assembly of the nuclear pore – biochemically singled-out steps revealed with NEM, GTP–gamma–S, and BAPTA. J Cell Biol 1996, 132:five–20.
-
Goldberg MW, Wiese C, Allen TD, Wilson KL: Dimples, pores, star–rings, and thin rings on growing nuclear envelopes: evidence for structural intermediates in nuclear pore circuitous assembly. J Prison cell Sci 1997, 110:409–420.
-
Smythe C, Jenkins HE, Hutchison CJ: Incorporation of the nuclear pore handbasket poly peptide Nup153 into nuclear pore structures is dependent upon lamina assembly: show from jail cell–costless extracts of Xenopus eggs. EMBO J 2000, 19:3918–3931.
-
Walther TC, Fornerod M, Pickersgill H, Goldberg Grand, Allen TD, Mattaj IW: The nucleoporin Nup153 is required for nuclear basket formation, nuclear pore complex anchoring and import of a subset of nuclear proteins. EMBO J 2001, xx:5703–5714.
-
Lutzmann M, Kunze R, Buerer A, Aebi U, Hurt E: Modular self–assembly of a Y–shaped multiprotein circuitous from seven nucleoporins. EMBO J 2002, 21:387–397.
-
Wente SR, Gasser SM, Caplan AJ: The nucleus and nucleocytoplasmic ship in Saccharomyces cerevisiae. In: The Molecular and Cellular Biology of the Yeast Saccharomyces (Edited by: JR Broach, Eastward Jones, J Pringle). Common cold Spring Harbor, New York: Cold Spring Harbor Laboratory Printing; 1997, 3:471–546.
-
Doye V, Hurt E: From nucleoporins to nuclear pore complexes. Curr Opin Jail cell Biol 1997, nine:401–411.
-
Gomez–Ospina N, Thousand Morgan, Giddings J, T. H., Kosova B, Hurt E, Winey One thousand: Yeast nuclear pore complex assembly defects determined past nuclear envelope reconstruction. J Struct Biol 2000, 132:1–5.
-
Marelli M, Lusk CP, Chan H, Aitchison JD, Wozniak RW: A link betwixt the synthesis of nucleoporins and the biogenesis of the nuclear envelope. J Jail cell Biol 2001, 153:709–723.
-
Bucci M, Wente SR: In vivo dynamics of nuclear pore complexes in yeast. J Prison cell Biol 1997, 136:1185–1199.
-
Bucci G, Wente SR: A novel fluorescence–based genetic strategy identifies mutants of Saccharomyces cerevisiae defective for nucler pore complex assembly. Mol Biol Cell 1998, ix:2439–2461.
-
Grandi P, Schlaich N, Tekotte H, Hurt EC: Functional interaction of Nic96p with a cadre nucleoporin complex consisting of Nsp1p, Nup49p and a novel protein Nup57p. EMBO J 1995, xiv:76–87.
-
Schlaich NL, Haner Grand, Lustig A, Aebi U, Injure EC: In vitro reconstitution of a heterotrimeric nucleoporin complex consisting of recombinant Nsp1p, Nup49p, and Nup57p. Mol Biol Prison cell 1997, viii:33–46.
-
Grandi P, Doye Five, Injure EC: Purification of NSP1 reveals complex formation with 'GLFG' nucleoporins and a novel nuclear pore protein NIC96. EMBO J 1993, 12:3061–71.
-
Kosova B, Pante Due north, Rollenhagen C, Hurt Due east: Nup192p is a conserved nucleoporin with a preferential location at the inner side of the nuclear membrane. J Biol Chem 1999, 274:22646–22651.
-
Aitchison JD, Rout MP, Marelli One thousand, Blobel G, Wozniak RW: Two novel related yeast nucleoporins Nup170p and Nup157p – complementation with the vertebrate homologue Nup155p and functional interactions with the yeast nuclear pore–membrane protein Pom152p. J Cell Biol 1995, 131:1133–1148.
-
Kenna MA, Petranka JG, Reilly JL, Davis LI: Yeast N1e3p/Nup170p is required for normal stoichiometry of FG nucleoporins within the nuclear pore complex. Mol Cell Biol 1996, xvi:2025–2036.
-
Marelli M, Aitchison JD, Wozniak RW: Specific binding of the karyopherin Kap121p to a subunit of the nuclear pore complex containing Nup53p, Nup59p, and Nup170p. J Cell Biol 1998, 143:1813–1830.
-
Tcheperegine SE, Marelli M, Wozniak RW: Topology and functional domains of the yeast pore membrane protein Pom152p. J Biol Chem 1999, 274:5252–5258.
-
Zabel U, Doye V, Tekotte H, Wepf R, Grandi P, Hurt EC: Nic96p is required for nuclear pore formation and functionally interacts with a novel nucleoporin, Nup188p. J Cell Biol 1996, 133:1141–1152.
-
Wente SR, Blobel Yard: A temperature–sensitive NUP116 cipher mutant forms a nuclear envelope seal over the yeast nuclear pore complex thereby blocking nucleocytoplasmic traffic. J Cell Biol 1993, 123:275–84.
-
Novick P, Field C, Schekman R: Identification of 23 complementation groups required for post–translational events in the yeast secretory pathway. Jail cell 1980, 21:205–215.
-
Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama NR, Rexach MF, Ravazzola M, Amherdt 1000, Schekman R: COPII: a membrane coat formed past Sec proteins that drive vesicle budding from the endoplasmic reticulum. Prison cell 1994, 77:895–907.
-
Kaiser CA, Ferro–Novick S: Send from the endoplasmic reticulum to the Golgi. Curr Opin Cell Biol 1998, x:477–482.
-
Siniossoglou Southward, Wimmer C, Rieger Thou, Doye V, Tekotte H, Weise C, Emig Due south, Segref A, Hurt EC: A novel circuitous of nucleoporins, which includes Sec13p and a Sec13p homolog, is essential for normal nuclear pores. Jail cell 1996, 84:265–275.
-
Siniossoglou South, Lutzmann M, Santos–Rosa H, Leonard Yard, Mueller S, Aebi U, Hurt E: Structure and assembly of the Nup84p complex. J Cell Biol 2000, 149:41–53.
-
Allen NPC, Huang L, Burlingame A, Rexach M: Proteomic analysis of nucleoporin interacting proteins. J Biol Chem 2001, 276:29268–29274.
-
Yoshihisa T, Barlowe C, Schekman R: Requirement for a GTPase–activating poly peptide in vesicle budding from the endoplasmic reticulum. Scientific discipline 1993, 259:1466–1468.
-
Kaiser CA, Schekman R: Singled-out sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell 1990, 61:723–733.
-
Rose Md, Misra LM, Vogel JP: KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell 1989, 57:1211–1221.
-
Wente SR, Rout MP, Blobel G: A new family unit of yeast nuclear pore circuitous proteins. J Cell Biol 1992, 119:705–723.
-
Sacher M, Jiang Y, Barrowman J, Scarpa A, Burston J, Zhang L, Schieltz D, Yates JRI, Abeliovich H, S Ferro–Novick: TRAPP, a highly conserved novel complex on the cis–Golgi that mediates docking and fusion. EMBO J 1998, 17:2494–2503.
-
Sacher Thousand, Barrowman J, Wang Westward, Horecka J, Pypaert Grand, Ferro–Novick Southward: TRAPP I implicated in the specificity of tethering in ER–to Golgi transport. Mol Cell 2001, vii:433–442.
-
Duden R, Hosobuchi M, Hamamoto S, Winey M, Byers B, Schekman R: Yeast β– and β'– coat proteins (COP). J Biol Chem 1994, 269:24486–24495.
-
Barlowe C: Traffic COPs of the early secretory pathway. Traffic 2000, ane:371–377.
-
Rossi G, Kolstad K, Stone S, Palluault F, Ferro–Novick S: BET3 encodes a novel hydrophilic protein that acts in conjuction with yeast SNAREs. Mol Biol Cell 1995, 6:1769–1780.
-
Potenza 1000, Bowser R, Muller H, Novick P: SEC6 encodes an 85 kDa protein required for exocytosis in yeast. Yeast 1992, 8:549–558.
-
Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P: Coatomer is essential for retrieval of dilysine–tagged proteins to the endoplasmic reticulum. Jail cell 1994, 79:1199–1207.
-
Trumbly RJ: Glucose repression in the yeast Saccharomyces cerevisiae. Mol Microbiol 1992, 6:15–21.
-
Matynia A, Salus SS, Sazer S: Iii proteins required for early steps in the secretory pathway also affect nuclear envelope strucure and cell cycle progression in fission yeast. J Cell Sci 2002, 115:421–431.
-
Yamanushi T, Hirata A, Oka T, Nakano A: Characterization of yeast sar1 temperature–sensitive mutants, which are defective in protein transport from the endoplasmic reticulum. J Biochem 1996, 120:452–458.
-
Wuestehube LJ, Duden R, Eun A, Hamamoto S, Korn P, Ram R, Schekman R: New mutants of Saccharomyces cerevisiae affected in the send of proteins from the endoplasmic reticulum to the Golgi complex. Genetics 1996, 142:393–406.
-
Kurihara T, Hamamoto S, Gimeno RE, Kaiser CA, Schekman R, Yoshihisa T: Sec24p and Iss1p function interchangeably in transport vesicle formation from the endoplasmic reticulum in Saccharomyces cerevisiae. Mol Biol Jail cell 2000, 11:983–998.
-
Higashio H, Kimata Y, Kiriyama T, Hirata A, Kohno Yard: Sfb2, a yeast poly peptide related to Sec24p, can function as a constituent of COPII coats required for vesicle budding from the endoplasmic reticulum. J Biol Chem 2000, 275:17900.
-
Nanduri J, Mitra S, Andrei C, Liu Y, Yu Y, Hitomi 1000, Tartakoff AM: An unexpected link between the secretory path and the system of the nucleus. J Biol Chem 1999, 274:33785–33789.
-
Nanduri J, Tartakoff AM: The arrest of secretion response in yeast: signaling from the secretoty path to the nucleus via Wsc proteins and Pkc1p. Mol Cell 2001, viii:281–289.
-
Sherman F, Fink GR, Hicks JB: Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1986.
-
Ito H, Fukuda Y, Murata K, Kimura A: Transformation of intact yeast cells treated with alkali cations. J Bacteriol 1983, 153:163–168.
-
Baudin A, Ozier KO, Denouel A, Lacroute F, Cullin C: A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res 1993, 21:3329–3330.
-
Heim R, Tsien RY: Engineering green fluorescent protein for improved effulgence, longer wavelengths and fluorescence resonance energy transfer. Curr Biol 1996, vi:178–182.
-
Sikorski RS, Hieter P: A system of shuttle vectors and yeast host strains designed for efficient manipulation of Deoxyribonucleic acid in Saccharomyces cerevisiae. Genetics1989, 122:xix–27.
-
Lawrence CW: Classical mutagenesis techniques. In: Guide to yeast genetics and molecular biology (Edited by: C Guthrie, GR Fink, vol). San Diego, CA: Bookish Printing, Inc 1991, 194:273–280.
-
Davis LI, Blobel G: Identification and characterization of a nuclear pore complex poly peptide. Cell 1986, 45:699–709.
-
Strambio–de–Castillia C, Blobel G, Rout MP: Isolation and characterization of nuclear envelopes from the yeast Saccharomyces. J Jail cell Biol 1995, 131:19–31.
-
Goldstein A, Lampen JO: β–D–fructofuronoside fructohydrolase from yeast. Methods Enzymol 1975, 42C:504–511.
-
Hubbard EJ, Yang XL, Carlson M: Relationship of the cAMP–dependent protein kinase pathway to the SNF1 protein and invertase expression in Saccharomyces cerevisiae. Genetics 1992, 130:71–80.
Acknowledgements
We wish to thank C. Hardy, C. Kaiser, A. Nakano, P. Novik, and R. Schekman for yeast strains; M. Rose for anti-Kar2 antibodies; Chiliad. Rout, C. Strambio-de-Castillia and G. Blobel for the anti-Pom152p monoclonal; A. Nett for help with cloning, and members of the Wente lab for disquisitional discussion. This work is supported by funds from the National Institutes of Health (GM20308 to K.J.R., and R01 GM57438 to S.R.West).
Author information
Affiliations
Corresponding writer
Rights and permissions
About this article
Cite this article
Ryan, One thousand.J., Wente, S.R. Isolation and characterization of new Saccharomyces cerevisiae mutants perturbed in nuclear pore circuitous assembly. BMC Genet 3, 17 (2002). https://doi.org/x.1186/1471-2156-3-17
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/1471-2156-three-17
Keywords
- Green Fluorescence Protein
- Nuclear Envelope
- Complementation Group
- Endoplasmic Reticulum Network
- Temperature Sensitive Phenotype
Source: https://bmcgenomdata.biomedcentral.com/articles/10.1186/1471-2156-3-17
Belum ada Komentar untuk "Can 2 Different Ts Alleles of Sar1 Each Generate a Different Sec Phenotype?"
Posting Komentar